The EU has always been one of the core market targets of various medical device manufacturers. To obtain access to the EU market, it is necessary to meet the requirements of EU IVDR/MDR regulations.
On May 5, 2017, the EU In Vitro Diagnostic Medical Device Regulation EU 2017/746 (IVDR) was officially released and came into effect, and it will be implemented on May 26, 2022. From the date of implementation, IVDR will completely replace the original EU In vitro Diagnostic Devices Directive (IVDD). The new IVDR regulations have added a series of new requirements, including product classification, unique device identification (UDI), CE-related certifiers (manufacturers, EU authorized representatives, distributors, etc.) registration requirements in EU, performance evaluation and listing performance tracking requirements, Post-listing market surveillance requirements, etc. For manufacturers, the increased entry barriers to the EU market and its stricter regulation have brought great challenges to manufacturers.
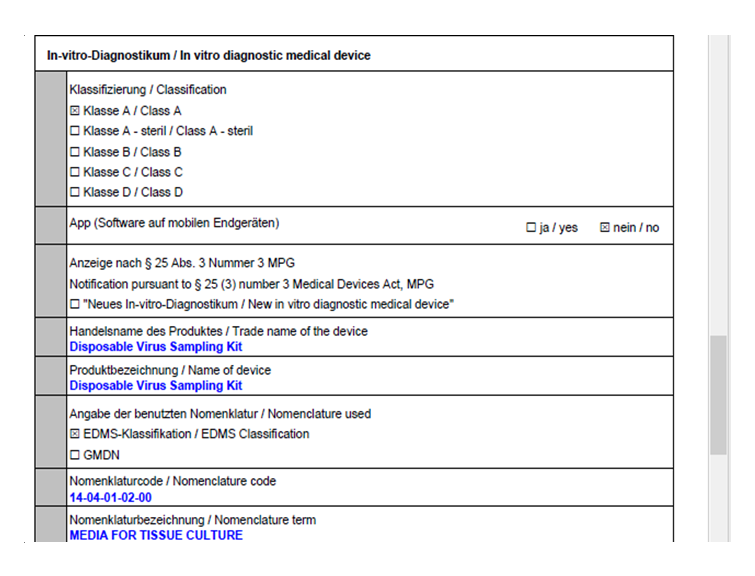
Wuhan EasyDiagnosis has carried out the regulatory tracking and implementation work intensively, in accordance with the requirements of IVDR regulations, improved product performance, completed product technical documents and system upgrades, 4 products: Disposable virus sampling tube, Sample release Reagent, Full-Automatic Nucleic acid Extraction and Purification Instrument and Nucleic Acid (DNA/RNA) Extraction Kit have passed EU IVDR registration on March 18, 2022.
Attached is a screenshot of the registration confirmation part: